Understanding Why Graphite Conducts Electricity
Graphite, a form of carbon, is known for its exceptional electrical conductivity. The material’s unique properties make it an essential component in various industries that rely on its conductive nature. But what makes graphite so proficient at conducting electricity?
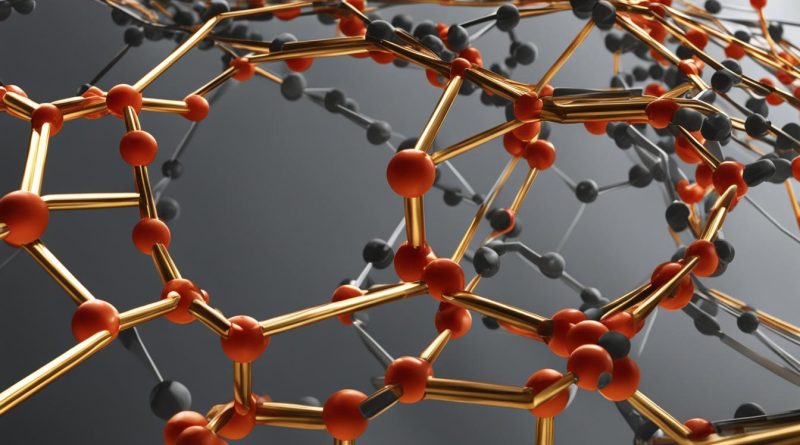
The answer lies in its structure and electron arrangement. Graphite has a layered crystal structure with hexagonally arranged carbon atoms and delocalized electrons that enable it to transfer electrons effectively. As a result, graphite’s electronic properties make it a vital component in industries such as electronics, energy, and lubrication.
Key Takeaways
- Graphite has a unique structure and electron arrangement that enable it to conduct electricity efficiently.
- The number of layers and purity of graphite can impact its conductivity.
- Graphite is essential in various industries due to its conductive properties.
- Understanding the mechanisms behind graphite’s conductivity is crucial for its application in energy production, electronics, and lubrication.
Graphite’s Structure and Electrical Conductivity
Graphite is a crystalline form of carbon with a unique structure that allows it to conduct electricity exceptionally well. Its conductivity is due to the arrangement of carbon atoms in its layers.
Each layer in its hexagonal crystal lattice is held together by covalently bonded carbon atoms, forming an endless flat sheet. These sheets are stacked on top of each other and separated by a distance of around 0.34 nm, forming thin layers. The layers are then held together by weak van der Waals forces, which makes them easily slide past one another. This arrangement of the carbon atoms creates a perfect pathway for electron movement, giving graphite its excellent electrical conductivity.
Graphite’s conductivity mechanism is based on its delocalized electrons. These electrons are not bound to individual atoms but move freely across the layers.
The number of layers present in graphite can impact conductivity. The greater the number of layers, the higher the conductivity due to the increased pathway for electron movement.

Factors Influencing Graphite’s Conductivity
Graphite’s conductivity is determined by several influencing factors. One of these factors is the number of layers present in the graphite structure. Generally, graphite with more layers tends to exhibit higher conductivity. This is because the increased number of layers provides more pathways for electron movement, allowing for improved conductivity throughout the crystal structure.
Another factor that impacts graphite’s conductivity is the purity of the material. Impurities or defects in the crystal structure can hinder the movement of electrons and reduce conductivity. This is particularly important in industrial applications that require high conductivity graphite, such as in electronics and batteries, where it is crucial to maintain a high level of purity to ensure maximum performance.
It is also worth noting the conductive nature of graphite, which has implications for its use in various industries. Due to its excellent electrical conduction properties, graphite is commonly utilized in the manufacturing of electrodes, where it can transfer electrical energy with ease. Its conductive nature also makes it a popular choice in lubricants, where it can help to dissipate heat and reduce friction between surfaces.
Conclusion
Graphite is an extraordinary material with exceptional electrical conductivity that is widely used in various industries. Its unique properties make it an excellent conductor of electricity, which is primarily due to its layered crystal structure and the ability of electrons to move within these layers.
Through this article, we have explored the factors that influence graphite’s conductivity and how it can be used in numerous applications, including electronics, batteries, and lubricants. Understanding the reasons behind graphite’s conductivity is crucial for its effective use in these industries.
Graphite’s conductive nature makes it a valuable material in the manufacturing of electrical components. Its high thermal conductivity and lubricity also make it an excellent material for certain applications such as in the aerospace and automotive industries.
As research continues to uncover the undiscovered potential of graphite, it is clear that it will remain an integral component of the 21st-century industry.
FAQ
Why does graphite conduct electricity?
Graphite conducts electricity due to its unique structure and the presence of delocalized electrons. The layered crystal structure of graphite allows electrons to move freely within the layers, providing a clear pathway for electrical conduction.
What are the electrical properties of graphite?
Graphite possesses excellent electrical conductivity, making it an ideal material for various electrical applications. It has a low electrical resistance and can efficiently transfer electrons, enabling the flow of electric current.
How do the properties of graphite enable electrical conduction?
The layered crystal structure of graphite consists of hexagonally arranged carbon atoms bonded covalently. This arrangement forms a pathway for electron movement through the layers, allowing them to easily slide past one another. The presence of delocalized electrons within these layers enables efficient electrical conduction.
What factors influence graphite’s conductivity?
Several factors can impact the conductivity of graphite. One significant factor is the number of layers present in the graphite structure. Graphite with more layers tends to have higher conductivity due to an increased number of pathways for electron movement. Additionally, the purity of graphite plays a role, as impurities or defects in the crystal structure hinder electron movement and can reduce conductivity.
What are the applications of graphite’s electrical conductivity?
Graphite’s excellent electrical conductivity makes it valuable for a range of applications. It is used in electronics, such as circuit boards and electrodes, where the efficient transfer of electrons is crucial. Graphite also finds application in batteries, lubricants, and various other industries that require good electrical conductivity.
How does graphite’s conductivity compare to other materials?
Graphite exhibits higher electrical conductivity compared to many other non-metallic materials. However, its conductivity is lower than that of most metals. Nonetheless, its unique combination of electrical conductivity, along with other desirable properties like thermal stability and low friction, makes graphite a preferred choice in specific applications.